
Kathleen A. McDonough, PhD
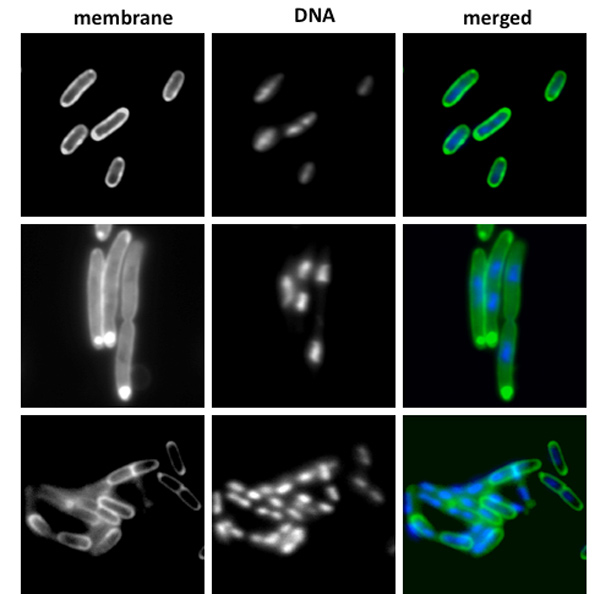
The focus of the McDonough Laboratory is gene regulation in the context of bacterial pathogenesis, or the means by which bacteria cause disease. The team is primarily interested in two well known pathogens: Mycobacterium tuberculosis, the bacterium that causes TB, and Yersinia pestis, the etiologic agent of bubonic and pneumonic plague. The lab uses a variety of techniques in their studies with both pathogens, ranging from molecular genetics and biochemistry to bioinformatics, proteomics and fluorescence microscopy.
The long term objective of the laboratory’s Mtb work is to gain a better understanding, at the molecular and cellular levels, of how this highly pathogenic bacterium establishes infection so that effective strategies can be developed to prevent tuberculosis infection and/or disease. A major current emphasis in the laboratory is on Mtb gene expression in host-associated conditions, and this work encompasses two major projects. The first project focuses on cyclic AMP (cAMP) signaling as a global gene regulatory mechanism, while the second involves the characterization of a family of macrophage-induced genes that are thought to play a role in the establishment of TB dormancy. A long term goal of the Yersinia program is to understand the specific roles of gene regulation in plague transmission and pathogenesis as Y. pestis cycles between its flea vectors and mammalian hosts. A second objective of the Yersinia work is to understand, at the cellular and molecular levels, what differentiates Y. pestis, the etiologic agent of plague, from the closely related enteric pathogen Y. pseudotuberculosis.
To learn more, please visit the McDonough Laboratory.